FDA accepts Merck's Remicade biopharmaceutical listing application
May 26, 2016 Source: Bio Valley
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];US pharmaceutical giant Merck & Co recently announced that the FDA has accepted the biologics license application (BLA) for biosimilar SB2 (infliximab, infliximab) submitted by partner Samsung Bioepis (Samsung Bioepis). The drug is a biosimilar of Johnson & Johnson (JNJ) heavy brand drug Remicade (like grams, generic name: infliximab, infliximab). Remicade is the world's best-selling anti-inflammatory drug with global sales of $9.24 billion in 2014.
The BLA is the first biosimilar BLA submitted by both parties in the US market, with the aim of seeking FDA approval for all currently approved treatment indications for Remicade. Merck said that the FDA's acceptance of SB2's BLA is an exciting milestone for the company's collaboration with Samsung Bioepis in the biosimilars sector. If approved, SB2 will provide an important treatment option that will help meet the needs of US physicians, patients, and healthcare systems for affordable, high-quality biosimilars.
Samsung Bioepis is a joint venture between Samsung Electronics (Samsung Biologics), a subsidiary of South Korean electronics giant Samsung, and Biogen, a US biotechnology giant, in 2012. It is committed to the development of high-quality biosimilars, including Samsung Bio. The formulation holds 85% of the shares.
Merck has entered into a partnership with Samsung Bioepis in 2013. The biosimilars developed by the two companies cover immunology, oncology and diabetes. Five products are in Phase III clinical trials, including: SB2 (Remicade, gram) and SB3 (Herceptin). , Herceptin), SB4 (Enbrel, Enli), SB5 (Humira, Xiu Mei Le), SB9 (Lantus, when to come). Both parties have plans to submit all five generic applications for listing to the global regulatory authorities during 2015-2016.
At present, Samsung Bioepis has reached the following related agreements with Merck on the commercialization of biosimilar products in the fields of immunology, oncology and diabetes:
Merck:
—— SB4 (Enbrel Biosimilar), responsible for the region: global scope (excluding the United States, the European Union, Switzerland, Japan);
—— SB2 (Remecade), responsible for the region: global scope (including the United States, but not including the EU, Switzerland, Russia, Turkey);
—— SB5 (Humira Biosimilar ), responsible for the region: global scope (including the United States, but not including the EU, Switzerland, Russia, Turkey);
—— SB3 (Herceptin Biosimilar), responsible for the region: global scope;
—— SB9 (Lantus Biosimilar), responsible for the region: global scope.
MLPA ( Multiplex Ligation-dependent Probe Amplification) method detects multiple copy number changes of genes or loci sites. Nowadays, MLPA is used to check large numbers of hereditary disorders and tumour profiling.Since Dutch Scientist Jan Schouten first invented it, the MLPA method was first published in 2002 'Nucleic Acid Research'. The principle of the MLPA is to apply the specific probe design targeting a region of interest on each sample DNA. MLPA method consists of the following steps:
Denaturation > Hybridization Ligation > PCR MLPA Amplificons Capillary Electrophoresis >Data Analysis
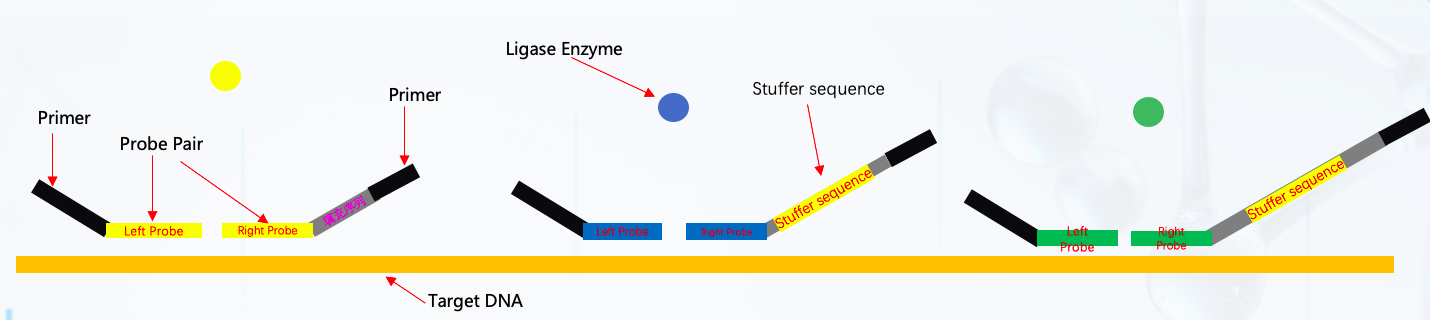
PCR reaction consists of the three steps: denaturation, primer annealing and primer elongation. Those steps of PCR amplification are repeated many times. The fluorescence-labeled primers, which will go through the capillary electrophoresis to pass a detector, are incorporated into the size of the amplification products. The measured fluorescence was visualized as a peak pattern, the so-called electropherogram. The raw data from the capillary electrophoresis analyzer forms the input of the MLPA analysis.
The genetic analyzers of Superyears Gene can be used not only for Sanger sequencing but also for fragment analysis. Fragment analysis First, it obtains the fluorescence-labelled DNA fragments, performs the capillary electrophoresis using a Genetic Analyzer and compares the sample's relative size standard with the designed size standard markers through the analysis software.
The genetic analyzer of Superyears Gene can be used in the capillary electrophoresis during the process of the MLPA experiment, and the data obtained can be used for (compatible) professional analysis software.
Genetic Analyzer For Mlpa,3130Xl Genetic Analyzer,Genetic Analyzer Capillary Electrophoresis,Genetic Analyzer Gene Sequencing Mlpa
Nanjing Superyears Gene Technology Co., Ltd. , https://www.superyearsglobal.com