Top 5 research and development progress worthy of attention in the field of immuno-tumor in 2018
January 15, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];In 2017, immuno-oncology dominated the “headline†position of the FDA and major cancer conferences. Two CAR-T (chimeric antigen T cell receptor) products were approved by the FDA - Novartis' Kymriah for leukemia and Gilead/Kite's Yescarta for lymphoma. These two products provide some patients with the ability to reprogram immune cells to identify and attack cancer. Some immunosuppressive "checkpoint" drugs, such as Keytruda from Merck (MSD), have also received FDA approval, which provides new treatment options for cancer patients.
So what are the developments in immuno-oncology that are worthy of attention in 2018? Let us take a sneak peek.
1. CAR-T treatment beyond leukemia and lymphoma
Now Novartis and Gilead (acquisition of Yescarta developer Kite Pharma) have demonstrated the feasibility of producing personalized immune cell therapies for individual patients, and some companies and academic groups are working to make the technology available to more people. Among them was the start-up company Bluebird Bio, which announced in December that 17 of the 18 patients who participated in the multiple myeloma CAR-T trial responded well to treatment.

The treatment, called bb212, is being developed by Celgene and Bluebird Bio and is currently undergoing Phase 2 clinical trials. Recently, the treatment has been approved by the FDA's “breakthrough†therapy, which will have Help accelerate the process of research and development.
But can CAR-T technology also be applied to solid tumors? Novartis and Kite Pharma are studying this possibility, and small companies such as Celyad in Belgium and Bellicum Pharmaceuticals in Houston are also studying this possibility. Celyad recently began a CAR-T trial in colorectal cancer. Bellicum is undergoing a CAR-T trial in pancreatic tumors and is expected to have preliminary results by 2018.
2. Juno Therapeutics turns around in the CAR-T field
2016 was a tough year for Juno Therapeutics, which was once recognized as a major player in the CAR-T field but was forced to withdraw due to side effects in clinical trials. Things turned around in 2017, especially in December, when the company showed the latest clinical data from its leading CAR-T therapy, JCAR017. The data show that the therapy achieves a 68% complete response rate in the treatment of aggressive B-cell non-Hodgkin's lymphoma with a lower incidence of relative side effects. Juno has pledged to apply for FDA approval in the second half of 2018.

3. The research and development of oncolytic virus therapy is in full swing
Many viruses have the ability to naturally kill cancer, and some companies have been researching engineered viruses to target certain tumor types. Amgen received its first FDA approval for melanoma in the field of its oncolytic virus, Imlygic, an engineered form of herpes. Since then, this field has been relatively calm, but it is expected to have further development this year.
A recent concern in the field of oncolytic viruses is the UK's PsiOxus Therapeutics, which has developed oncolytic adenovirus therapy in collaboration with Bristol-Myers Squibb (BMS). The treatment, called NG-348, uses a virus to transfer two therapeutic genes directly into a tumor, thereby recruiting immune cells to attack cancer. In December, the company announced that it began human trials in solid tumors, which resulted in a $15 million milestone payment from BMS.
Other companies of concern include Viralytics, which is investigating several tumor types of coxsackie virus (more commonly the common cold), and Oncolytics Biotech is conducting advanced clinical trials of reovirus-preferred metastatic breast cancer.
4. Innovation portfolio development process
The rise of immuno-oncology has led to better predictions of immuno-enhancement therapy in combination therapy – whether it is with similar drugs or with old treatments like chemotherapy. In 2017, MSD's PD-1 mAb Keytruda combined with the chemotherapy drugs pemetrexed and carboplatin was approved for non-squamous non-small cell lung cancer (NSCLC).
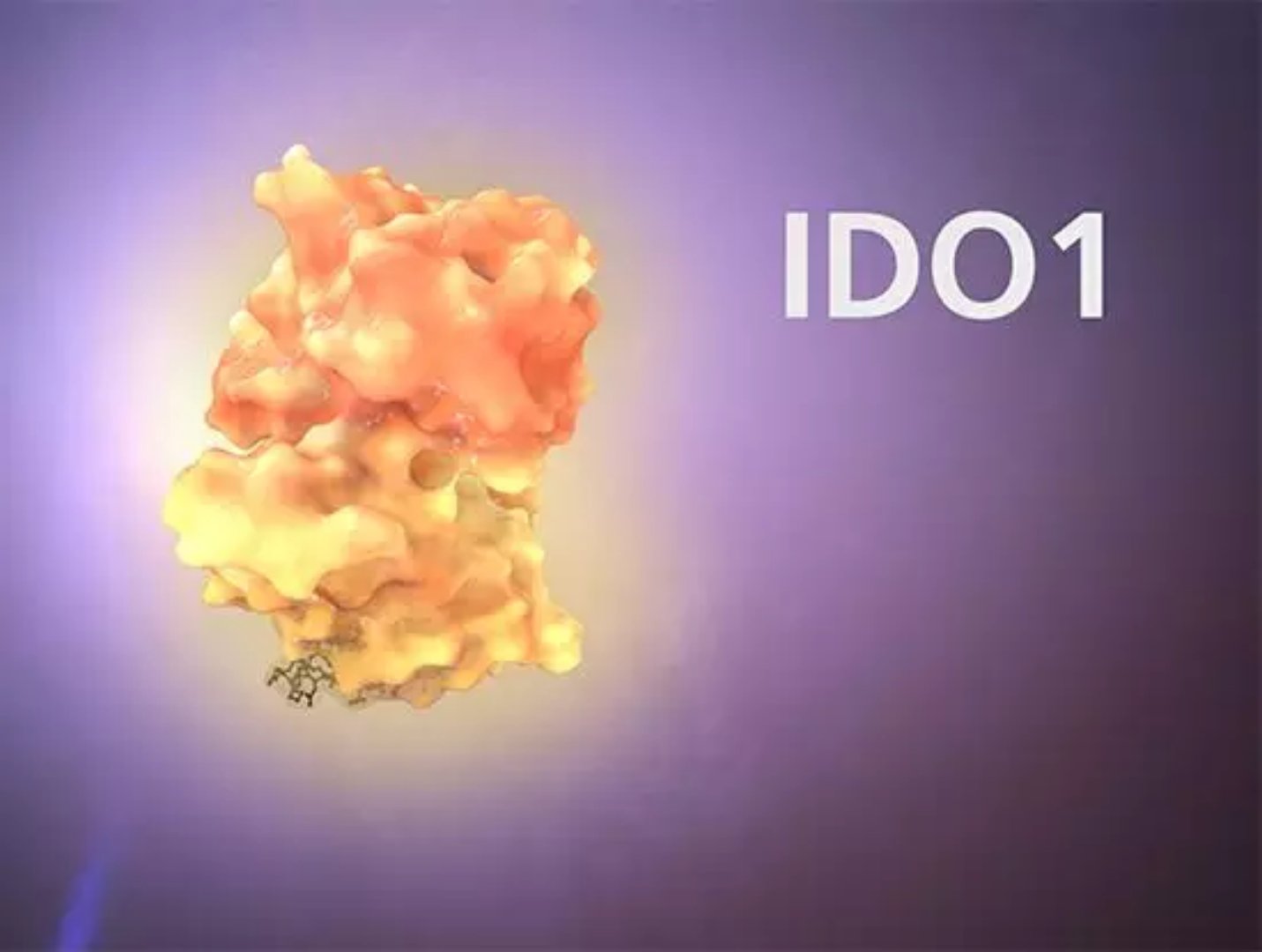
â–² Epacadostat inhibits the IDO1 enzyme in the human body, a key enzyme that suppresses the immune system and promotes the regulation of T cell production and inhibits the activation of effector T cells. (Source: Incyte)
Look for more such innovative combinations this year to get key test data. For example, several drugs are being used in conjunction with Incyte's epacadostat, including Keytruda (melanoma), BMS Opdivo (lung cancer and head and neck cancer), and AstraZeneca's Imfinzi (lung cancer). Epacadostat is the first (first-in-class) highly potent selective oral inhibitor in the field of IDO1 enzymes, which reverses tumor-associated immunosuppression and restores an effective anti-tumor immune response.
5. The development of immuno-oncology promotes mergers and acquisitions
Speaking of Incyte, the progress it has made so far in joint trials has made it the first M&A target. Analysts have seen Gilead as a potential acquirer (before Gilead spent $11.9 billion to win Kite Pharma). Despite this, Incyte is expected to remain the target of the acquisition due to its research and development in IDO1 inhibitors.

Juno and bluebird are also frequent visitors to the list of analysts listed. And now that the US tax reform has passed, the company's overseas cash return costs have been reduced – many biopharmaceutical companies have assets to make major acquisitions. These include Pfizer, Amgen ($39 billion) and Merck ($20 billion) holding $22 billion in overseas cash.
Analysts predict that even Gilead, which won the biggest deal in the field of immune oncology in 2017, could use its $32 billion in cash to build its immune-enhanced cancer product pipeline.
Reference materials:
[1] Forbes: 5 Immuno-Oncology Developments To Watch In 2018
Herb extract we mainly supply are monk fruit extract, shiitake mushroom extract, reshi mushroom extract and other mushroom extract like lion`s mane extract. The main active ingredient among those powders are polysaccharides, beta glucan and so on. And monk fruit extract is one of our hot selling natural sweetener which can be added in very wide range of food and beverage. Unlike sugar, it is a kind of non- nutritive sweetener which is low in calories and high in sweetness, welcome to reach us for more information.
Herb Extract,Reishi Mushroom Extract,Mushroom Extract,Shiitake Mushroom Extract
YT(Xi'an) Biochem Co., Ltd. , https://www.ytwholefood.com