PFS doubles! Tumor electric field therapy announced 2 excellent results
June 05, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Today, Novocure, a biotechnology company dedicated to the use of electric fields to treat tumors, announced at the ASCO annual meeting in 2018 that the company's cancer treatment electric field combined with standard chemotherapy for the treatment of patients with recurrent ovarian cancer clinical phase 2 test results. The results of the trial showed that the combination of tumor treatment electric field and paclitaxel combined with paclitaxel monotherapy increased the progression free survival (PFS) of patients by more than 2 times.

Ovarian cancer is one of the most important cancers that cause death in women. It is the type of cancer that causes the most female deaths in female reproductive system cancer.
Tumor therapy developed by Novocure does not rely on chemicals or biological agents, but uses an electric field that is transformed at a specific frequency. The company's original tumor treatment electric field is capable of changing the polarity of the electric field at a frequency of 100,000 to 300,000 times per second. Tumor cells undergo mitosis, and tubulin and septin in the cells need to be moved to specific locations within the cell to ensure successful mitosis. These two proteins are polar proteins that carry both positive and negative charges, and their movement is affected by the tumor treatment electric field. Tumor treatment electric fields disrupt tumor cell division by preventing these proteins from moving to the correct location within the cell.
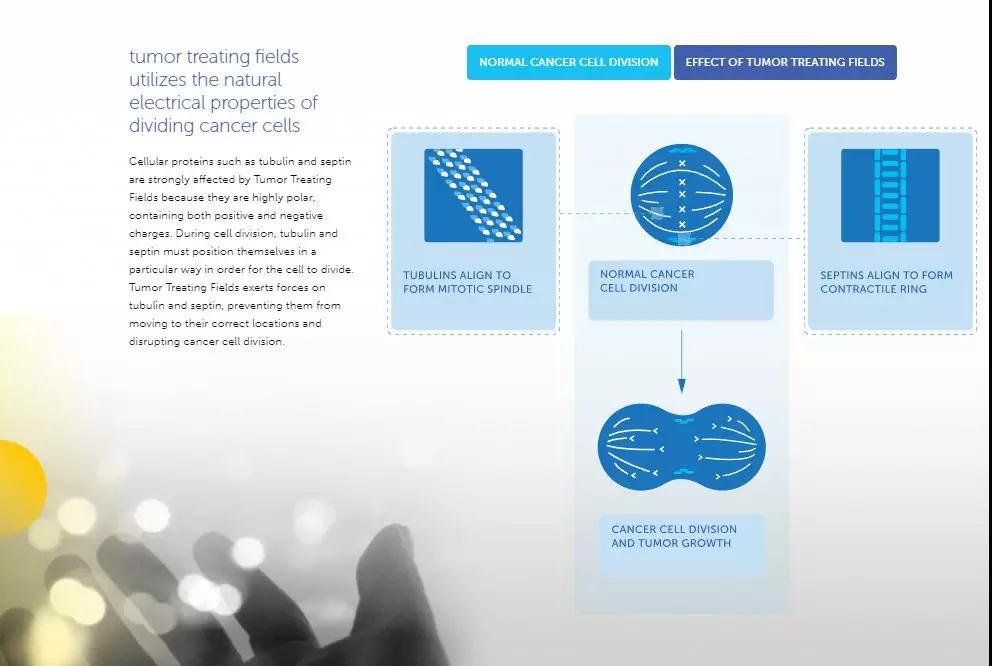
â–²The mechanism of action of this therapy (Source: Novocure official website)
The tumor treatment electric field does not stimulate human tissue or cause tissue fever, and its frequency can be adjusted to target only certain size tumor cells. It has very little damage to healthy cells. The most common side effect is mild or moderate skin irritation.
In an open-label, open-label, multicenter, single-arm clinical phase 2 trial with a historical control, 30 patients with recurrent ovarian cancer underwent treatment with a combination of a tumor treatment electric field and paclitaxel. Data from the paclitaxel control group in the bevacizumab clinical phase 3 trial was used as a historical control for this clinical phase 2 trial.

â–²Novocure Executive Chairman Bill Doyle pointed out at the 2018 WuXi PharmaTech Global Forum that the treatment of solid tumors will change radically in the future.
The trial results showed that patients treated with combination therapy had an average PFS of 8.9 months, whereas the historically controlled paclitaxel monotherapy group had a PFS of 3.9 months. As of the publication of the results, the average 1-year survival rate of the patient population was 61%. Efficacy studies have shown that combination therapy with tumor treatment electric field and paclitaxel can increase PFS by more than 2 times and improve overall survival of patients compared with paclitaxel monotherapy. Safety results indicate that this combination therapy is well tolerated and safe and can be used as a first-line therapy for patients with recurrent ovarian cancer.
At the same time, Novocure announced the trial design of the company's upcoming clinical Phase III trial called INNOVATE-3. INNOCATE-3 is a prospective, open-label trial that will include 540 patients with recurrent ovarian cancer who are resistant to platinum-based therapy. Patients will be randomized to receive paclitaxel monotherapy or combination therapy with paclitaxel and a tumor treatment electric field at a frequency of 200 kHz. The primary end point of the study was overall survival, with secondary endpoints being PFS, objective response rate, severity of side effects, and frequency of occurrence.
"After the recurrence of ovarian cancer, the average overall survival of patients is only 13 to 14 months," said Dr. Eilon Kirson, chief scientific officer and head of research and development at Novocure. "Our preclinical studies have shown that tumors treat electric fields and taxanes. There is a complementary role between chemotherapy. We are pleased to announce the clinical phase 3 trial design of INNOVAT-3 at the ASCO annual meeting, and we are eager for patients who need better therapy to join this clinical trial."
Reference materials:
[1] Novocure to Present INNOVATE-3 Phase 3 Pivotal Trial Design in Recurrent Ovarian Cancer at the American Society of Clinical Oncology Annual Meeting 2018
[2] Novocure official website
Tetanus Shot,Tetanus Vaccine,Hepatitis B Injection,Hep B Vaccine
FOSHAN PHARMA CO., LTD. , https://www.foshanmedicine.com