China's first drug-reported CAR-T therapy clinical trial application accepted
December 12, 2017 Source: Bio Discovery
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];On December 11, a number of media reported that the LCAR-B38M CAR-T cell autologous reinfusion preparation of Nanjing Legend Biotechnology Co., Ltd. has submitted a clinical trial application according to the “New Class 1 drug for therapeutic use of biological productsâ€, and the acceptance number is CXSL1700201. It is reported that this is the first CAR-T therapy in China to report clinical trials based on drugs.
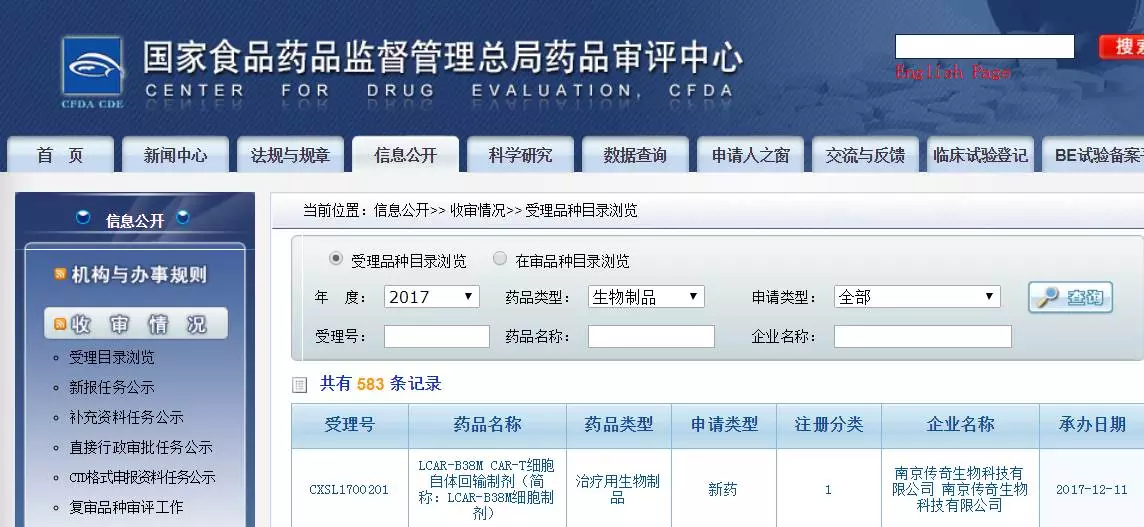
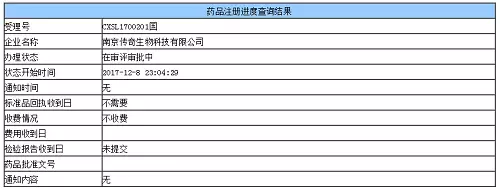
On October 23 this year, the CFDA General Office publicly solicited opinions on the Drug Registration Management Measures (Revised Draft). In the simultaneous publication of the "Biological Product Registration Classification and Application Requirements (Trial)", the applicant wrote that if the applicant wants to register the cell therapy products according to the drug, it can be declared according to the corresponding category requirements of the therapeutic biological products.
Among them, new gene therapy and cell therapy-based biological products (such as innovative mechanisms, new vectors, new target cells, etc.) should be declared in accordance with the registration classification. Gene therapy and cell therapy-based biological products that are improved on the basis of domestic and foreign listed products shall be declared in accordance with the classification of the registration category.
At the annual meeting of the American Society of Clinical Oncology (ASCO) held in June this year, the legendary LCAR-B38M of Nanjing has attracted worldwide attention for its amazing data. Data presented at the meeting showed that 35 patients who underwent LCAR-B38M treatment responded to LCAR-B38M treatment in a clinical study of LCAR-B38M in the treatment of refractory or relapsed multiple myeloma. The response rate is amazingly 100%. In addition, 19 patients had a median follow-up of 6 months, of which 18 patients had complete remission, 1 patient had partial remission, and LCAR-B38M treatment was also 100% effective. The result is the strength to crush Bluebird in the United States.
2017 has undoubtedly become a crucial year in the development of CAR-T therapy. In August and October of this year, the US FDA approved the launch of Novartis's world's first CAR-T product, Kymriah, and Kite Pharma's second CART-T therapy, Yescarta. It is worth mentioning that both Kymriah and Yescarta are patients whose T cells are designed to target a molecule called CD19 on the surface of cancer cells.
The official approval of these two products has brought greater confidence to domestic and foreign companies working on CAR-T therapy development, including the development of CAR-T therapy for other targets. LCAR-B38M is a CAR-T therapy targeting BCMA (CD269). The reason why BCMA is of great concern is that its RNA is almost always found in multiple myeloma cells, and this protein has also been found on the surface of malignant plasma cells in patients with multiple myeloma. Both of these factors indicate that BCMA is a target worth developing.
As a "living" drug, CAR-T therapy is very different from traditional drugs. To put it simply, this type of therapy separates T cells from patients and transforms them into T cells in vitro, with a "navigation" that specifically recognizes cancer cells, the chimeric antigen receptor (CAR). These "modified CAR-T cells" are then expanded and returned to the patient to exert a specific anticancer effect.
Some analysts said that the future market space for CAR-T therapy is expected to be between 35 billion and 100 billion US dollars. At present, the international companies in the forefront include Novartis, Kite Pharma, Cellectis, Juno and so on. In addition, giants including Pfizer, Servier, Shinki, Gilead are also actively involved in this field.
In recent years, a large number of companies that have joined the competition for CAR-T therapy have emerged in China. In addition to the earlier layout of Sibbiman, Boshiji, Koji Bio, Aikangde, etc., a large number of listed companies have joined the competition, including Galaxy Bio, Hengrui Medicine, Anke Bio, Zuoli Pharmaceutical, East Cheng Pharmaceutical, Zhongyuan Concord, Sansheng Pharmaceutical, Fosun Pharma, etc. With the more relevant domestic policies, we look forward to the early listing of CAR-T therapy in China, bringing more choices for cancer patients.
Part of this article refers to the self-medication Rubik's Cube, Medical Mike, Singularity Network, Biopharmaceutical Xiaobian.
Real Time PCR Detection System
Disease Detection System,Real Time Pcr Equipment,Pcr Amplification System,Nucleic Acid Detection System
Hangzhou DIAN Biotechnology Co., Ltd. , https://www.dianbiotech.com