With the increasing demand for domestic drinking water market in recent years, government departments are paying more and more attention to the sanitary status of bottled water. However, looking at the sample surveys in recent years in China, the situation is not optimistic. This year, the number of unqualified bottled waters jointly announced by the city's industrial and commercial bureaus and quality supervision bureaus has reached hundreds, involving 251 brands and more than 100 drinking water manufacturers.
Many problems such as the excessive number of drinking water colonies have been exposed, and many people are worried about being afraid. This is undoubtedly a wake-up call for every drinking water manufacturer. For questions such as the total number of drinking water colonies exceeding the standard, we consulted Mr. Zhou, the disinfection expert.
First of all, most of the drinking water is easily stored in a 5 gallon plastic bucket. Since the 5 gallon bucket is recycled and reused, it has high requirements for cleaning and disinfection. Therefore, once the disinfection is not complete, it is easy to cause secondary pollution. If the production technology and equipment are not closed, resulting in poor sealing, then after entering the storage and transportation, circulation, it is easy to cause the barrel cover to loosen, allowing air to enter, resulting in unqualified water quality.
Secondly, microorganisms such as the total number of colonies are important indicators reflecting the quality of drinking water. The level of total colony content indicates the extent to which drinking water is contaminated by microorganisms. Production processes, production environments, personnel hygiene, packaging containers, and merchandise transportation may cause bacterial contamination. In addition, poor sealing of the cap will also contaminate drinking water. Manufacturers should pay more attention to sterilization and disinfection in the production process and production. Rational use of existing sterilization technology for anti-virus, namely UV anti-virus, ozone static anti-virus, combined with NICOLER dynamic air anti-virus technology, comprehensive disinfection of equipment in the production workshop, and air. NICOLER dynamic air anti-virus technology adopts the latest NICOLER three-stage bidirectional plasma electrostatic field working principle. The disinfection process is: the high-voltage direct current pulse causes the plasma electrostatic field to generate a reverse electric effect, generating a large amount of plasma. Under the action of the negative pressure fan, the polluted air in the air is pumped into the machine, and the negatively charged bacteria are broken down by the plasma electrostatic field. The new mechanism is repeated three times to ensure the sterilization effect, and then the components such as the drug-impregnated activated carbon are combined. For secondary sterilization filtration, the treated clean air is circulated rapidly in large quantities, keeping the controlled environment in a “sterile and dust free†standard. Since the person can work in the workshop at the same time when the workshop is sterilized, the sterilizer is called "NICOLER sterilizer".
Drinking water production enterprises in the production process due to incomplete disinfection of barrels and lids, incomplete cleaning and disinfection of production equipment, or insufficient air cleaning in the filling workshop, improper management of operators, or production closures are not closed, It may cause the total number of bottled drinking water colonies to fail. Therefore, choose a reasonable sterilization method, so that enterprises can guarantee product quality and win a larger consumer market.
MLPA ( Multiplex Ligation-dependent Probe Amplification) method detects multiple copy number changes of genes or loci sites. Nowadays, MLPA is used to check large numbers of hereditary disorders and tumour profiling.Since Dutch Scientist Jan Schouten first invented it, the MLPA method was first published in 2002 'Nucleic Acid Research'. The principle of the MLPA is to apply the specific probe design targeting a region of interest on each sample DNA. MLPA method consists of the following steps:
Denaturation > Hybridization Ligation > PCR MLPA Amplificons Capillary Electrophoresis >Data Analysis
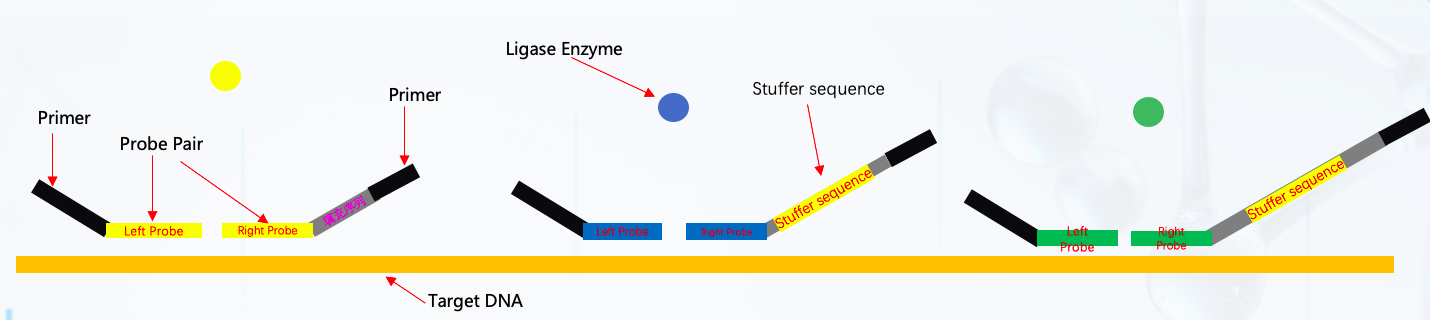
PCR reaction consists of the three steps: denaturation, primer annealing and primer elongation. Those steps of PCR amplification are repeated many times. The fluorescence-labeled primers, which will go through the capillary electrophoresis to pass a detector, are incorporated into the size of the amplification products. The measured fluorescence was visualized as a peak pattern, the so-called electropherogram. The raw data from the capillary electrophoresis analyzer forms the input of the MLPA analysis.
The genetic analyzers of Superyears Gene can be used not only for Sanger sequencing but also for fragment analysis. Fragment analysis First, it obtains the fluorescence-labelled DNA fragments, performs the capillary electrophoresis using a Genetic Analyzer and compares the sample's relative size standard with the designed size standard markers through the analysis software.
The genetic analyzer of Superyears Gene can be used in the capillary electrophoresis during the process of the MLPA experiment, and the data obtained can be used for (compatible) professional analysis software.
Genetic Analyzer For Mlpa,3130Xl Genetic Analyzer,Genetic Analyzer Capillary Electrophoresis,Genetic Analyzer Gene Sequencing Mlpa
Nanjing Superyears Gene Technology Co., Ltd. , https://www.superyearsglobal.com