Medical Network September 12th Recently, the State Drug Administration approved the marketing of furazolinib capsules, which will bring new treatments for patients with metastatic colorectal cancer. The treatment of advanced colorectal cancer is mainly chemotherapy, and more combined chemotherapy is used. Patients with colorectal cancer who failed first-line treatment were mainly treated with second-line chemotherapy, but the follow-up treatment plan for patients after the failure of current second-line standard treatment is still lacking. Furazolinib is a highly selective long-acting inhibitor of VEGFR. Indications include colorectal cancer and non-small cell lung cancer. Its appearance can be said to break the chemotherapy-based situation of advanced colorectal cancer, which may give the late stage. The survival of colorectal cancer brings new benefits. The furazolinib group benefited significantly regardless of whether it had previously received anti-VEGF or anti-EGFR therapy. At the same time, the safety of furazolinib is good. In conclusion, furazolinib can be one of the standard treatments for advanced colorectal cancer in third-line treatment.
Since the launch of the first fully-anti-cancer drug, apatinib, which was launched in China at the end of 2014, the development of new anticancer drugs in China has been increasing year by year, and a lot of new drugs have emerged, from conventional chemotherapy to stepwise precision treatment. Targeted therapy, and now the most advanced immunotherapy, advanced cancer patients face more choices. According to relevant data, China Medical Industry Information Center has sorted out the routine process of applying for approval for new drug research and development to the listing stage, as shown in the following table:
Table 1. Regular process for registration and approval of domestic new drug research and development to the listing stage
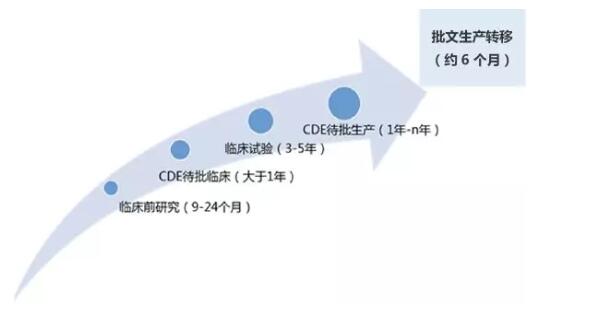
Source: China Pharmaceutical Industry Information Center
Since 2017, CDE has accelerated the speed of listing and approval of major new drugs. It takes about one year or less from the time when the new drug is filed and the drug is officially listed. Based on the public information of CDE and the CPM of China New Drug Development Monitoring Database, we predict that these anticancer drugs will be listed in China in the next six months. The treatment scope covers common cancers such as colon cancer, liver cancer, breast cancer and lymphoma.
  Table 2. Domestic anti-cancer heavy new drugs coming soon
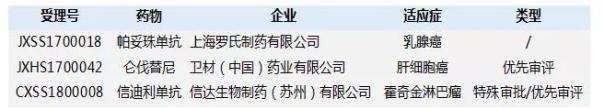
Source: China Pharmaceutical Industry Information Center China New Drug Development Monitoring Database CPM
Anti-tumor targeted therapy drugs listed and listed in China in 2018
The targeted therapeutic drugs listed in China in 2018 include the treatment of non-small cell lung cancer, erlotinib HCl, the world's first ovarian cancer PARP inhibitor olapaini, and the self-developed breast cancer class 1.1 EGFR/HER2 inhibition in China. A solution of pyrrolidine maleate and a newly approved drug for the treatment of metastatic colorectal cancer, furazolinib. In addition, the following heavy new drugs are also receiving attention.
Luvutinib
On November 3, 2017, the market application for the treatment of hepatocellular carcinoma with lenvatinib mesylate was accepted by CDE. In December 2017, it was included in the CDE for priority review and has now reached the CFDA approval stage.

Luvutinib is a multi-target receptor tyrosine kinase inhibitor independently developed by Eisai Co., Ltd., which was approved by the US FDA and the European EMA Authority for invasive, locally advanced or metastatic differentiated thyroid in 2015. In the treatment of cancer, in 2016, FDA and EMA successively approved the combination of rivastatinib and everolimus for the treatment of advanced renal cell carcinoma.
At this year's ASCO annual meeting, a heavy published study showed that the overall survival of the rivastatinib group was not inferior to sorafenib (13.6 months vs. 12.3 months), reaching the primary end point of the study.
Pertuzumab
On January 2, 2018, the application for the breast cancer drug patenuzumab in China was accepted by CDE and has been approved by the CFDA. It has now reached the review stage of pharmaceutical research.
Pertuzumab was first marketed in the United States in June 2012. It has now become a HER2-amplified breast cancer patient with a full-course medication option (preoperative neoadjuvant can be used, postoperative adjuvant therapy can be used, advanced treatment) Can also be used).
Compared with Herceptin combined with chemotherapy, pertuzumab combined with Herceptin can significantly improve the efficiency and prolong survival. A phase III clinical trial of treatment with pertuzumab + Herceptin + docetaxel showed a median overall survival of 56.5 months in the Pattuzin group and 40.8 months in the control group. 15.7 months. The addition of a drug has extended the overall survival of patients with advanced breast cancer by an average of more than a year. It is an epoch-making "miracle."
Trastuzumab + Pertuzumab + Chemotherapy has been approved by more than 75 countries worldwide for neoadjuvant therapy for HER2-positive breast cancer and is the standard of care for HER2-positive breast cancer patients.
Currently listed and upcoming anti-tumor immunotherapy drugs in China
The marketing application for PD-1 monoclonal antibody Opdivo (Navuliyub) is accepted by CDE. This makes Opdivo the first PD-1/PD-L1 drug listed in China. Citrix monoclonal antibody is the second PD-1/PD-L1 drug to be applied for in China after Opdivo, and is the first domestic PD-1 monoclonal antibody application.
Sindi monoclonal antibody
On December 13, 2017, the State Food and Drug Administration accepted the application for the listing of Cinda Biological's domestic PD-1 inhibitor, Xindi monoclonal antibody injection. If successfully approved, this will become China's first domestically produced PD-1 inhibitor. It is also the second PD-1/PD-L1 drug to be applied for in China after Opdivo. After resubmission, it will enter the CFDA review stage.

Xindi monoclonal antibody injection code IBI308, the application for the indication is Hodgkin's lymphoma. In addition to this indication, IBI308 is also conducting clinical studies of non-small cell lung cancer, esophageal cancer, and NK/T cell lymphoma, primarily for the treatment of advanced solid tumors.
The domestic layout of small molecule targeted anti-tumor drugs is still dominated by international giants.
According to Chinese medicine industry information center statistics, SFDA approved a total of 20 kinds of targeted anti-cancer drugs, of which more than 80 percent of the original research imported drugs, and more for small molecule drugs; only four kinds of Chinese pharmaceutical companies to develop drugs for new drugs: Among them, nimotuzumab is the first functional monoclonal antibody for the treatment of malignant tumors in China; ectinib is the first small molecule targeted anti-tumor drug with complete independent intellectual property rights in China; The drug's apatinib is the world's first small molecule anti-VEGFR-2 targeted drug that has been proven to be safe and effective after the failure of standard chemotherapy for advanced gastric cancer; the core biotin cidabenamine is the world's first approved histone. The acetylase inhibitor is also the first original innovative drug licensed in the United States and other developed countries for the treatment of recurrent or refractory peripheral T-cell lymphoma. Overall, companies that are currently applying for anti-tumor targeted small molecule drugs in China in 2018 are exclusively owned by Roche.
Table 3. Domestically approved anti-cancer drug targeting small molecules up to the current 2018
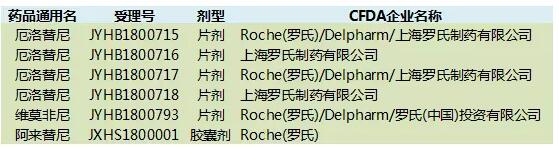
Source: China Pharmaceutical Industry Information Center China New Drug Development Monitoring Database CPM
The new pattern of immunotherapy in China brings many opportunities and challenges
In recent years, cancer immunotherapy has attracted much attention, and as related research continues to deepen, PD-1 inhibitors have gradually demonstrated their potential for cancer treatment. The miracle drug Pabolimumab is the "endorsement" of President Carter of the United States. PD-1 inhibitors aim to fight tumors through the body's own immune system, with a variety of tumor indications, and are expected to substantially improve patient survival.
The approved Pabolizumab is the PD-1 inhibitor K drug we often call. As a heavy drug for immunotherapy, Pabolizumab is only approved for the treatment of advanced melanoma patients in China. On the top, Pabolizumab currently has the widest range of indications and is the world's first broad-spectrum anticancer drug.
Merck's Pabolivumab was listed in China more than a month later than Opdivo, but the indications for the two were different. Domestically approved Opdivo is used for second-line treatment of non-small cell lung cancer, while rudder is used for locally advanced or metastatic melanoma that progresses after first-line treatment.
A new era of immunotherapy in China is about to open. At the same time, the FDA has adopted an accelerated approval policy for innovative drugs, breakthrough drugs and rare diseases, and has also rapidly promoted the market for innovative drugs that are urgently needed. Under the policy dividend, who will dominate the future market for cancer?
Sun Shade Netting,Shade Netting For Vegetables,Green Sun Shade Net,Green Net For Sun Protection
Changzhou Satidi Import and Export Co., Ltd. , https://www.guanjiejts.com